Introduction
Materials and Methods
Sampling and morphological study
Molecular protocol
Results
Morphology
Molecular data
Discussion
Introduction
The plant bug family Miridae (Insecta: Hemiptera: Heteroptera) is the largest group within the true bugs, comprising over 11,000 described species (Schuh and Weirauch, 2020). As their common name suggests, most species in Miridae are phytophagous and live on various plants; thus, many groups are recognized as insect pests in both forestry and agriculture (Wheeler, 2000a; Schuh and Weirauch, 2020). On the other hand, some groups are highly important in agriculture as biological control agents, making the family Miridae economically significant (Wheeler, 2000b).
The genus Proboscidocoris Reuter, 1882 is one of the large groups within the subfamily Mirinae, with a total of 55 described species and its members are broadly distributed in the world, from the Afrotropical, Oriental, and Palaearctic Regions (Schuh, 2002-2013). The host plants are currently recognized in only several species, and are unavailable in the most cases, but most of them are low-growing herbaceous plants (e.g., Lamiaceae, and Poaceae).
In Korea, the genus Proboscidocoris is currently represented by a single recorded species, P. varicornis. This species is commonly found throughout the Korean Peninsula and is particularly abundant in herbaceous plants near agricultural fields. This species is frequently observed feeding on herbaceous plants, and its wide host range suggests that it may play a role as a pest in agricultural ecosystems (direct observation).
This species has been originally described from Korea (Jakovlev, 1904). After that, Josifov and Kerzhner (1972) has suggested that it may be conspecific to P. malayus, which was later recorded in India and Vietnam. However, since the type specimen of P. malayus was unavailable at that time and research in India and Vietnam is not still ongoing (see details in Kerzhner and Josifov, 1999), further investigation is needed to determine whether P. varicornis is indeed the same species as P. malayus, or to verify any differences between the Korean population and those from Vietnam and India.
This study included the collection of samples from the genus Proboscidocoris over several years in both the Korean Peninsula and Vietnam. We conducted a taxonomic study between the Proboscidocoris species from these two regions based on morphological data and molecular marker.
Materials and Methods
Sampling and morphological study
The Proboscidocoris species in type localities, in Korea and in Vietnam were directly collected by the authors (Table 1). The specimens were examined and compared under the light stereomicroscope Nikon SMZ 800, combined with a camera BKONV300. For examination of male genitalia, the genital segment was detached from the abdomen, and was heated in 10% KOH solution at 70℃ for 5 min until it became transparent. The segment was washed in distilled water and was dissected to male genital structures. Terminology mainly follows Kim and Jung (2019). Depository of the specimen is at the Laboratory of Systematic Entomology, Chungnam National University, Daejeon, Korea (CNU).
Table 1.
Detailed information of Proboscidocoris varicornis.
Molecular protocol
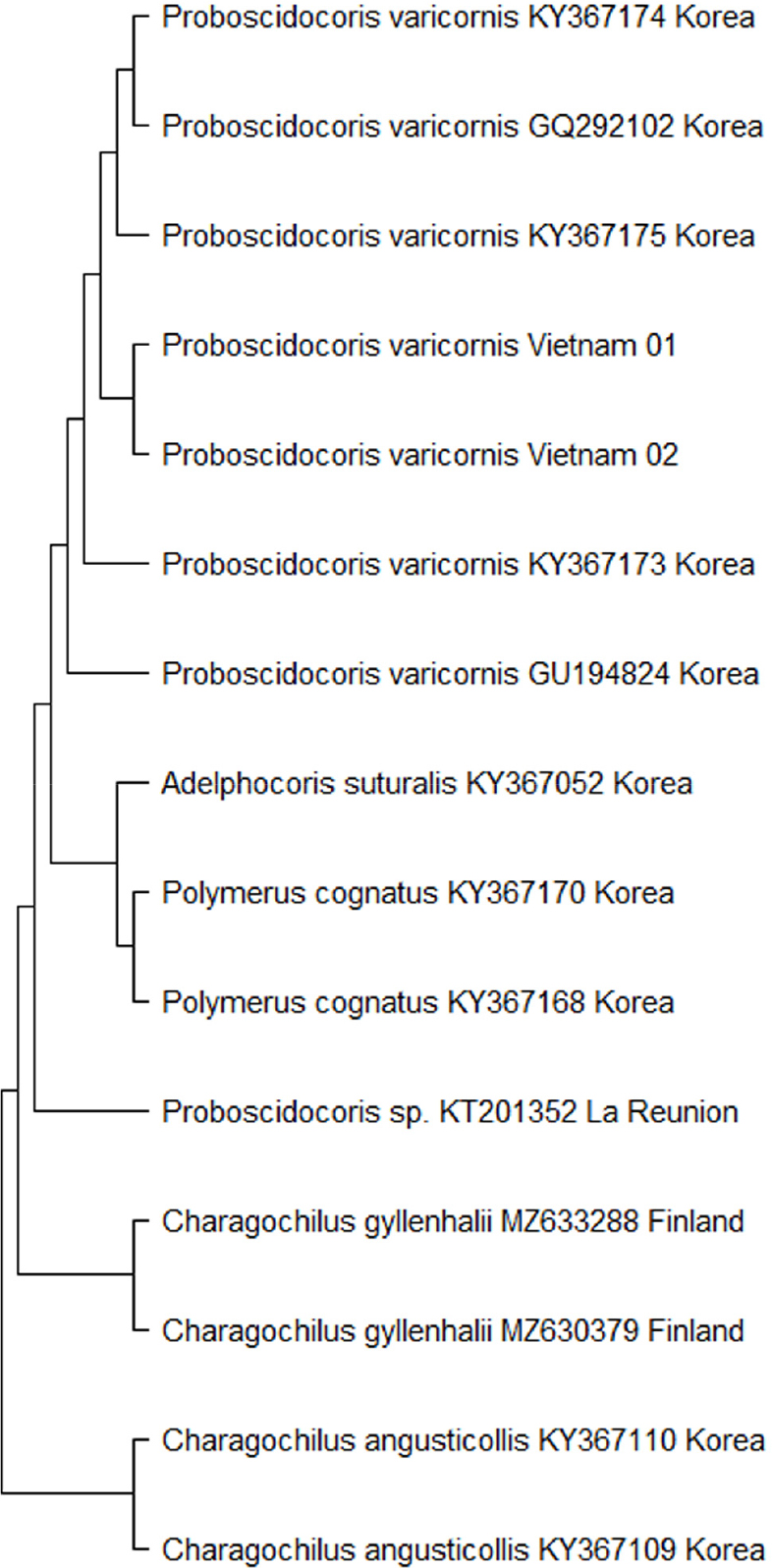
Genomic DNA was extracted the rest of the abdomen excluding the genital segment during the morphological preparation, using a QIAamp DNA Mini Kit in accordance with the manufacturer’s protocol (Qiagen, Germany). PCR was performed using the Solg 2X Taq PCR pre-mix (SolGent, Korea) with the primer set LCO1490 (GGT CAA CAA ATC ATA AAG ATA TTG G) and HCO2190 (TAA ACT TCA GGG TGA CAA AAA ATC A) (Folmer et al., 1994). The taxa included in this analysis were selected based on the phylogenetic results for the subfamily Mirinae (Kim and Jung, 2019), with a focus on their close phylogenetic relationships to the genus Proboscidocoris. The majority of the sequences used were derived from previous molecular studies by the same authors (Kim and Jung, 2018, 2019). Sequences from the Vietnamese population were generated specially for this study, while additional sequences were obtained from public database (NCBI) (see Table 2). A simply modified neighbor-joining tree (NJ) (Saitou and Nei, 1987), was constructed, which is a commonly employed method for molecular diagnosis. Adelphocoris suturalis (Jakovlev, 1882) was used as the outgroup. All samples are deposited in CNU as voucher specimens.
Table 2.
Information of samples for molecular work used in this study.
| Species | Collecting site | GenBank accession number | References |
| Adelphocoris suturalis | Korea | KY367052 | Kim and Jung (2018) |
| Charagochilus angusticollis | KY367109 | ||
| KY367110 | |||
| Charagochilus gyllenhalii | Finland | MZ633288 | |
| MZ630379 | |||
| Polymerus cognatus | Korea | KY367168 | |
| KY367170 | |||
| Proboscidocoris varicornis | KY367173 | ||
| KY367174 | |||
| KY367175 | |||
| GU194824 | Jung and Lee (2012) | ||
| GQ292102 | Jung et al. (2011) | ||
| Vietnam | PQ643315 | Sequenced for this study | |
| PQ643316 | |||
| Proboscidocoris sp. | La Reunion | KT201352 | Atiama et al. (2017) |
Results
Morphology
The external morphology and genital structures of P. varicornis from both populations exhibited no differences (Fig. 1). Consistent with the Korean populations, the specimens from Vietnam displayed variation in dorsal coloration. However, no variation was observed in the parameres and endosoma (Fig. 1B-D and F-H).
Molecular data
In the simplified NJ analysis, the molecular sequences of P. varicornis were nearly identical, with a range of 0 - 0.1%. Sequences from each population clustered into distinct clades (Fig. 2). The interspecific genetic distance between the two Proboscidocoris species (P. varicornis and Proboscidocoris sp.) was found to be 15%, a value that is sufficiently distinct to support their separation at the species level, based on previous barcode study of the family Miridae (Kim and Jung, 2018). In case of the genus Charagochilus, the interspecific genetic distance between the two species (C. angusticollis and C. gyllenhalii) was approximately 17%, which was similar at the species level (Table 3).
Table 3.
Pairwise distances of the sequences used in this study.
Discussion
Based on morphological and molecular data, it is confirmed that the Proboscidocoris specimens collected from Korea and Vietnam are conspecific. The Proboscidocoris specimens from Korea are identical to the type specimen of P. varicornis, and since the type locality of P. varicornis is also Korea, it follows that the species collected from Vietnam are also P. varicornis. This finding supports the recent study (unpublished) documenting the first record of P. varicornis in Vietnam. However, the genetic distance between P. varicornis and P. sp. was found to be approximately 15%. We obtained the sequence data of P. sp. from NCBI and we suggested that this species was misidentified and/or the detailed taxonomic study of P. sp. is needed.
In this study, the Vietnamese congener, P. malayus was not detected, and its type specimens remain unavailable, as noted by Kerzhner and Josifov (1999). Consequently, without examination of P. malayus, we were unable to address the taxonomic issue regarding the synonymy between P. varicornis and the latter described species, P. malayus. Nevertheless, the data generated in this study may be valuable for future research, particularly molecular studies, once specimens become available and their molecular data can be obtained. Recent molecular research has demonstrated that DNA can be successfully extracted from old dried specimens, which supports the potential utility of these future analyses (Namyatova et al., 2020).