Introduction
Materials and Methods
Experiment design, diets, and housing
Sample collection and analysis
Data analysis
Results and Discussion
Conclusion
Introduction
Weaning is a critical period in pig production (Sampath et al., 2022). During weaning, piglets’ gastrointestinal tract and immune system are not fully developed, besides piglets face multiple stressors, such as separation from their mother, feed changes (from milk to solid feed) and pathogen threats that often leads to diarrhea, low feed utilization could cause stunted growth and significant loss in pig production (Sampath et al., 2022). The inclusion of antibiotics and zinc oxide (ZnO) in weanlings diet become a common method to cope with this stressor. With the widespread ban on antibiotic growth promoters in feed, producers started to focus on the use pharmacological doses of ZnO (e.g., 2,000 mg·kg-1 or higher) in piglet diet to maintain growth and mitigate incidence of diarrhea during the post-weaning stage (Biswas et al., 2024). Though pharmacological dose control diarrhea, the high doses and/or prolonged use of 2,000 -3,000 ppm of ZnO in pigs has raised several concerns, such as the potential onset of toxic effects due to the accumulation of ZnO in their tissue (Burrough et al., 2019). Concurrently, increasing concerns regarding the antimicrobial-resistance threats led to mandate a future free from pharmacological levels of ZnO. Thus, finding suitable alternative additives to replace ZnO has become a focus point for many researchers.
Among alternative candidates’ organic acids (OA) and their salts have been accepted as one of the best in-feed additives globally (Upadhaya et al., 2016) due to their antimicrobial activity. Like antibiotics, OA can penetrate the bacterial cell wall and interrupt the normal actions of Salmonella spp., Escherichia coli, Clostridia spp. and some coliforms bacteria in host by reducing their gastro-intestinal pH (Suiryanrayna and Ramana, 2015). Apart from OA, essential oils (EO), which is aromatic, volatile and extracted from plant liquids also served as a promising feed additive due to their antibacterial and antioxidant properties with low toxicity (Shao et al., 2023). Especially, carvacrol, thymol, and cinnamaldehyde are widely used in animal feedstuffs. Previously, several studies have pointed out the beneficial effect of EO in swine production. For instance, Tian and Piao (2019) reported that blend of EO decrease the diarrhea incidence in weaning pigs by improving the antioxidative capability in their gut. Similarly, Xu et al. (2018) pointed out that combination of thymol and carvacrol increased growth performance, digestibility, and duodenal villus height in weaned piglets. Concurrently, the functional feed additive capsicum oleoresin (CO), a pungent component of chili peppers has the potential anti-inflammatory response that mediated at the transcriptional level within the lymphocytes of the gut mucosa (Nevius et al., 2012). In the earlier study, Liu et al. (2013) reported that inclusion of capsicum significantly decreases the intestinal expression of inflammatory genes and helps weanlings to improve their performance.
Swine feed industry is very familiar with the practice of feeding sweeteners. Sucralose is a non-nutritive artificial sweetener (AS) and is made from sucrose. Previously, Schiffman and Rother (2013) found that sucralose is six hundred times sweeter than sucrose and due to this reason sucralose has been widely used as a sweetener and flavor enhancer in the food industry. Zhang et al. (2020) reported that sucralose act as ideal feed additive for weanlings to improve growth performance by stimulating their feed intake. Similarly, Moran et al. (2010) reported that piglets fed with AS showed increased villus height, increased nutrient absorption, and improved tight junction strength. Based on the previous literatures, we hypothesized that replacing antibiotic and ZnO with nature additives such as OA, EO, CO, and AS might enhance the performance of weanlings. Though previous reports showed some positive effect on OA, blend of EO, CO, and AS in pigs, yet the combined effect of all these supplements in one weaning trial doesn’t exists. Thus, in this study we aimed to focus whether replacing high ZnO diet would reveal similar growth performance, nutrient digestibility, fecal microbial, and reduce diarrhea score in weaning when fed combination of nature additives (OAB, EO, CO, and AS).
Materials and Methods
The animal care procedures implemented in this study were approved (Approval No. DK-2-1941) by the Institutional Animal Care and Use Committee (IACUC) of Dankook University (Republic of Korea).
Experiment design, diets, and housing
At weaning d 21, a total of 200 ([Landrace × Yorkshire] × Duroc) piglets with an average body weight (BW) of 6.50 ± 0.66 kg were divided into 4 groups and assigned to have 1 of 5 different diets in a complete randomized block design. This trail falls into two phase feeding: Phase 1 (d 1 - 21) and phase 2 (d 22 - 42) and the mash form of basal diet were formulated to meet or exceed the nutrient requirements of NRC (2012) (Table 1). There were eight replicates with 5 pigs (2 female and 3 male) per pen. The test treatments were as follows:
1. Low ZnO (LZ) - basal diet + 125 ppm of ZnO;
2. High ZnO (HZ) - basal diet + 2,500 ppm of ZnO;
3. TRT 1 - LZ + 5 g·kg-1 OAB;
4. TRT 2 - LZ + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS;
5. TRT 3 - LZ + 5 g·kg-1 OAB + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS.
The main additive OAB (DaaFit Plus), EO (XTRACT® Caps XL), AS (Sucram C-150), CO (XTRACT® SHIELD) were commercially obtained from Pancosma R&D (Switzerland) and added to the experimental diets. The weaning facility was equipped with natural ventilation, slatted concrete floors, and the barn temperature was fixed at 26 - 28℃ with air humidity of 55 - 70%. Feeders and water dispensers were set in the corners of each pen and this arrangement provided unlimited access to both feed and water until day 42.
Table 1.
Basal diet composition for weaning pig (as fed-basis).
| Items (%) | Phase 1 | Phase 2 | ||||||||
| LZ | HZ | TRT1 | TRT2 | TRT 3 | LZ | HZ | TRT 1 | TRT 2 | TRT 3 | |
| Corn | 50.0675 | 49.49 | 50.0675 | 50.0375 | 50.0475 | 58.1175 | 57.53 | 58.0975 | 58.0775 | 58.0875 |
| SBM | 19.25 | 19.36 | 19.24 | 19.26 | 19.26 | 23.74 | 23.85 | 23.75 | 23.75 | 23.75 |
| FSBM | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 |
| SDPP | 3 | 3 | 3 | 3 | 3 | - | - | - | - | - |
| Tallow | 2.46 | 2.69 | 2.47 | 2.48 | 2.47 | 2.67 | 2.67 | 2.44 | 2.46 | 2.45 |
| Lactose | 7.78 | 7.78 | 7.78 | 7.78 | 7.78 | 3.18 | 3.18 | 3.18 | 3.18 | 3.18 |
| Sugar | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Whey protein | 7 | 7 | 7 | 7 | 7 | 3 | 3 | 3 | 3 | 3 |
| DCP | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.63 | 1.63 | 1.63 | 1.63 | 1.63 |
| Limestone | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 |
| Salt | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Met (99%) | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Lysine | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 |
| Mineral mixz | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin mixy | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Choline (25%) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| ZnO (80%) | 0.0125 | 0.25 | 0.0125 | 0.0125 | 0.0125 | 0.0125 | 0.25 | 0.0125 | 0.0125 | 0.0125 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated value | ||||||||||
| CP | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 |
| Ca | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| P | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 |
| Zn (ppm) | 17 | 2,017 | 117 | 117 | 117 | 17 | 2,017 | 117 | 117 | 117 |
| Lys | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Met | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| ME (kcal·kg-1) | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 |
| FAT | 4.81 | 5.03 | 4.82 | 4.84 | 4.83 | 4.81 | 5.03 | 4.82 | 4.84 | 4.83 |
| Lactose | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
LZ (low ZnO), basal diet + 125 ppm of ZnO; HZ (high ZnO), basal diet + 2,500 ppm of ZnO; TRT 1, LZ + 5 g·kg-1 OAB (organic acid blends); TRT 2, LZ + 0.025 g·kg-1 EO (essential oils) + 0.035 g·kg-1 CO (capsicum oleoresin) + 0.1 g·kg-1 AS (artificial sweetener); TRT 3, LZ + 5 g·kg-1 OAB + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS; SBM, soybean meal; FSBM, fermented soybean meal; SDPP, spray dried plasma protein; DCP, digestible crude protein; Met, methionine; CP, crude protein; ME, metabolizable energy; FAT, fat (total dietary fat content).
Sample collection and analysis
The BW of individual pigs were weighed at initial, and at the end of d 21, and 42. Feed allowance was recorded on a pen basis to calculate their average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G : F) at the end of phase 1, 2, and overall experimental period.
From d 35 to 42, 0.3% of chromium oxide (Cr2O3) was added to the experimental diet and collected in sterile bags for further analysis. The end of d 42, fresh fecal samples were randomly collected from 2 pigs/pen, pooled, taken to laboratory and stored at -20℃. Thawed feeds and fecal samples were weighed and subsequently dried at 105℃ for 3 consecutive days. Finally, feed and fecal samples were ground and sieved to pass through 1 mm-mesh sieve. The nutrient digestibility of dry matter (DM), nitrogen (N), and energy (E) were analyzed and the apparent total tract digestibility (ATTD) of nutrients was calculated according to Sampath et al. (2023).
At the end of d 21 and 42, fresh feces were collected from 2 pigs/pen using sterile tubes and immediately transported to the laboratory for microbial analysis. To confirm the existence of microbes, 1 gm of fresh specimen was taken and diluted 9 mL of 1% peptone broth and mixed well. Then 0.1% of peptone solution was poured into MacConkey (Difco Laboratories, USA) and Lactobacilli medium III (DSMZ, Germany) agar plates. The MacConkey agar plates were incubated at 37℃ for 1 day and Lactobacilli medium III agar plates were incubated at 39℃, for 2 days. The bacterial colonies were counted immediately taken out from the incubator.
The fecal scores were conducted from individual piglets twice daily at 09:00 and 16:00 during at initial, d 21, and d 42 of the experiment. The average stool score was noted on a pen basis every day. The fecal consistency of score was referenced on the 5-point scoring method of Sampath et al. (2022). In brief, the feces consistency scores criterion was as follows: Score 1 = hard, dry pellet; 2 = firm, formed stool; 3 = soft, moist stool that retains shape; 4 = soft, unformed stool that assumes shape of container; 5 = watery liquid that can be poured.
Data analysis
Experimental data was analyzed using general linear model (GLM) procedure of SAS ver. 9.2 (SAS Institute, USA) with pen as experimental unit. One-way ANOVA was performed and the significant difference was set at p < 0.05 and p < 0.10 as trend.
Results and Discussion
Early weaning always leads to decrease feed intake and growth rate, owing to poor digestibility and the palatability of solid diet. In this study, piglets fed diet supplemented with combination of natural additives like OAB, EO, CO, and AS showed significantly higher BW at d 42 (Fig. 1A) and significantly improved (p < 0.05) average daily gain (ADG) and reduced G : F at the end of d 42 and overall experimental period (Fig. 1B). The current findings were in line with Zhao et al. (2023) who observed increased ADG in pigs fed diet contain combination of thymol and cinnamaldehyde. On the other hand, Satessa et al. (2020) reported that inclusion of 2,500 mg·kg-1 ZnO in weaning diet had reduced the diarrhea score and improved ADG by lowering the feed conversion ratio (FCR). This finding was not agreed with our result in which HZ group pigs showed less performance compared to other group. A possible explanation for the increased BW and ADG might be related to the combination of experimental diets which helps to control the diarrhea incidence in pigs.
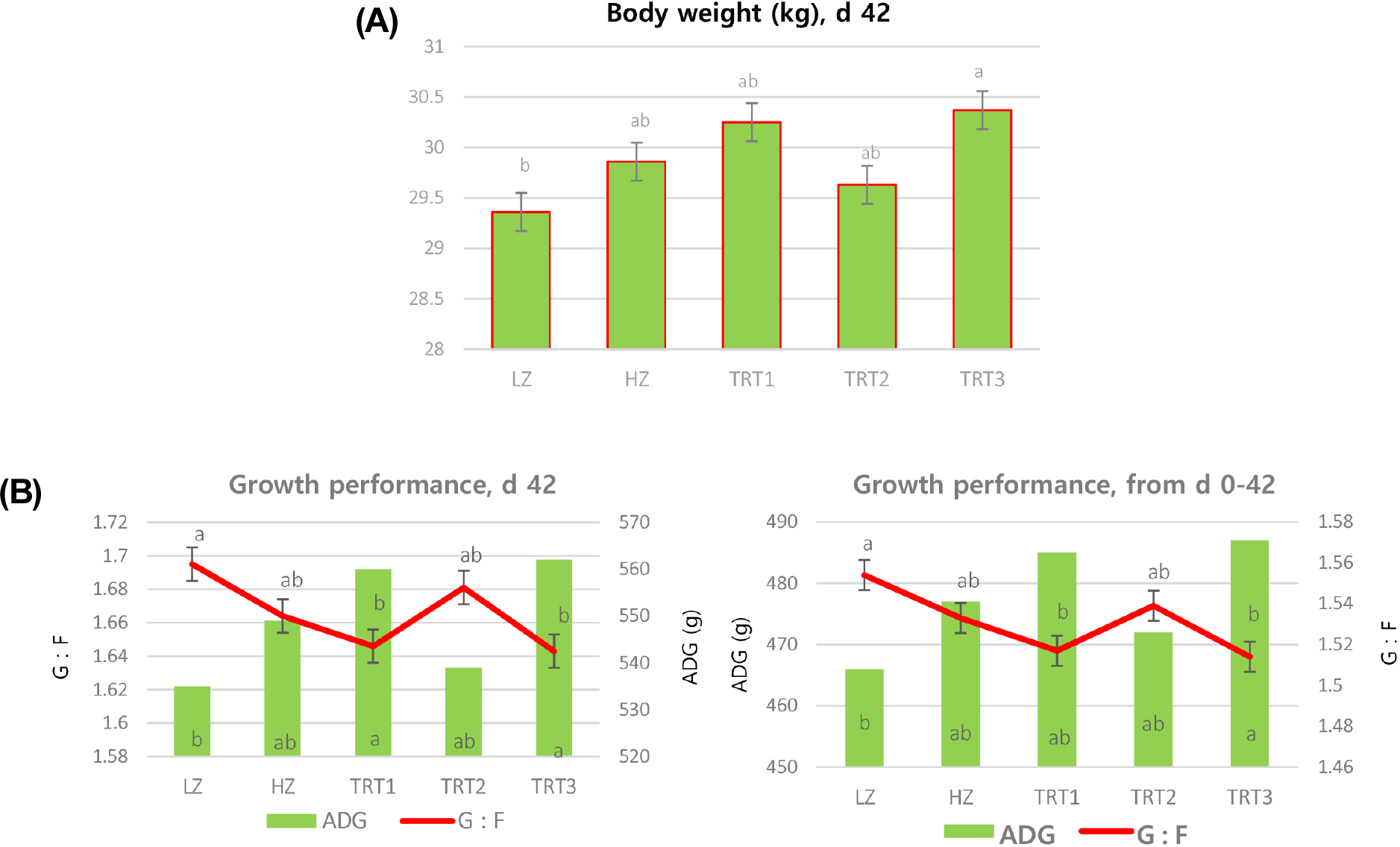
Fig. 1.
Effect of replacing zinc oxide (ZnO) with natural additives (organic acid blends [OAB], essential oils [EO], and capsicum oleoresin [CO]) on growth performance in weaning pigs. (A) Replacing ZnO with natural additives (OAB, EO, and CO) showed significantly increased body weight at d 42. (B) Replacing ZnO with natural additives (OAB, EO, and CO) showed significantly increased average daily gain (ADG) and reduced gain to feed ratio (G : F) in weanlings at the end of d 42 and overall experimental period. LZ (low ZnO), basal diet + 125 ppm of ZnO; HZ (high ZnO), basal diet + 2,500 ppm of ZnO; TRT 1, LZ + 5 g·kg-1 OAB; TRT 2, LZ + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS (artificial sweetener); TRT 3, LZ + 5 g·kg-1 OAB + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS.
The pH level of pigs is usually very high after weaning because of their limited hydrochloric acid (HCl) secretion ability caused by an immature gastrointestinal tract (GIT). Accordingly, protein digestibility may reduce when they started to had solid feed. Earlier, Ravindran and Kornegay (1993) and Partanen and Mroz (1999) reported that pigs fed diet supplemented with OAB reduces the passage rate of gastric digesta into the small intestine and stimulates enzyme secretion of the pancreas and help the host to increased digestibility of nutrients. Additionally, Amerah et al. (2011) reported that broilers had blend of EO (thymol and cinnamaldehyde) resulted in improved N digestibility. Similarly, Zitterl-Eglseer et al. (2008) reported a significant improvement of DM and crude protein digestibility by dietary supplementation of carvacrol and thymol EO. Thus, it was expected that the nutrient digestibility of DM, N, and E could be improved in pigs fed combination of natural additives. Unfortunately, there was no differences (p > 0.05) on nutrient digestibility of DM, N, and E in the current research (Table 2) which was not corroborate with Omogbenigun et al. (2003) who noted significant difference in DM digestibility in weanling fed diet supplemented with OAB. One possible reason for the lack of effects on nutrient digestibility in the current study could be due to the blend of different additives, dosage level, breed or age of animals.
EO could improve the ecology of the intestine by increasing the lactobacilli to enterobacteria ratio (Manzanilla et al., 2004). Previously, Xu et al. (2017) reported that weaning pigs fed diet supplemented with blend of EO and OAB has significantly increased Lactobacilli counts of feces (p < 0.05). However, there was no significant difference observed in the fecal microbiota of weaning pigs. The proposed reason for no significant changes of E. coli in the current study could be due to health status of animals or due to the hygiene status of the pig room which resulted with low diarrhea score (Table 2). Diarrhea is a severe problem in early stage of weaning pigs which leads to higher mortality rate. Previously, Fairbrother et al. (2005) stated that diets lacking in antibiotics caused an increased occurrence of diarrhea in pigs. Fortunately, there was no sign of fecal diarrhea score in the current study. Sutton et al. (1991) showed that diet supplementation with OAB or their salts had reduced the frequency of post weaning diarrhea and improve growth performance in piglets. A study by Diao et al. (2013) concluded that inclusion of 5,000 mg·kg-1 benzoic acid tended to decrease diarrhea in pigs, from this we proposed that benzoic acid may sustain the gastrointestinal tract environment and support the host to improve their growth performance.
Table 2.
Effect of replacing zinc oxide (ZnO) with natural additives on nutrient digestibility, fecal microbiota, and fecal score in weaning pigs.
| Items | LZ | HZ | TRT1 | TRT2 | TRT3 | SEM | p-valuey |
| Nutrient digestibility (%) | |||||||
| Day 42 | |||||||
| Dry matter | 80.90 | 80.45 | 81.54 | 81.39 | 81.19 | 2.11 | 0.995 |
| Nitrogen | 78.03 | 78.18 | 79.20 | 78.83 | 79.72 | 2.57 | 0.987 |
| Energy | 79.45 | 80.45 | 80.41 | 80.52 | 79.81 | 2.16 | 0.993 |
| Fecal microbiota (log10 cfu·g-1) | |||||||
| Day 21 | |||||||
| Lactobacillus | 7.37 | 7.36 | 7.31 | 7.33 | 7.35 | 0.03 | 0.681 |
| Escherichia coli | 6.12 | 6.15 | 6.13 | 6.10 | 6.11 | 0.04 | 0.921 |
| Day 42 | |||||||
| Lactobacillus | 7.42 | 7.41 | 7.39 | 7.37 | 7.36 | 0.04 | 0.762 |
| Escherichia coli | 6.10 | 6.15 | 6.18 | 6.12 | 6.14 | 0.05 | 0.780 |
| Fecal scorez | |||||||
| Day 7 | 3.25 | 3.29 | 3.25 | 3.27 | 3.27 | 0.03 | 0.917 |
| Day 21 | 3.20 | 3.21 | 3.18 | 3.20 | 3.21 | 0.02 | 0.815 |
| Day 42 | 3.14 | 3.16 | 3.13 | 3.14 | 3.16 | 0.04 | 0.972 |
LZ (low ZnO), basal diet + 125 ppm of ZnO; HZ (high ZnO), basal diet + 2,500 ppm of ZnO; TRT 1, LZ + 5 g·kg-1 OAB (organic acid blends); TRT 2, LZ + 0.025 g·kg-1 EO (essential oils) + 0.035 g·kg-1 CO (capsicum oleoresin) + 0.1 g·kg-1 AS (artificial sweetener); TRT 3, LZ + 5 g·kg-1 OAB + 0.025 g·kg-1 EO + 0.035 g·kg-1 CO + 0.1 g·kg-1 AS; SEM, standard error of the mean.
Conclusion
Addition of ZnO in pigs diet becomes a worldwide issue for animal production and may possess jeopardize for human health; thus, seeking alternatives ZnO have been greatly investigated recently. The current study demonstrates that replacing high ZnO with combination of natural additives (OAB, EO, and CO) could be beneficial to improve ADG and reduce G : F in weaning pigs, however, there were no effects observed on nutrient digestibility, fecal microbiota, and fecal score. We believe that current study would provide a novel insight to use natural additives to replace ZnO for sustainable pig production along with biosafety issues.




